News
Experts raise alarm, dengue rapid test kit withdrawn from Osu Sala
View(s):
Osu Sala has withdrawn the kit on the recommendation of the National Medicinal Drugs Authority (NMRA). Pic by Athula Devapriya
By Chrishanthi Christopher
The State Pharmaceuticals Corporation has decided not to sell the Dengue Rapid Diagnostic Kit (DRDK) at its Osu Sala outlets following complaints from health authorities that it will put patients at risk of developing the disease.
SPC Chairman Sarath Liyanage told the Sunday Times the decision to withdraw the kit a week after the sales began was taken on the recommendation by the National Medicinal Drugs Authority (NMRA).
The kit was sold at Rs. 620 at Osu Sala outlets and Osu Sala franchise holders throughout the country. The information leaflet in the pack carried the SPC logo. The leaflet claimed that the virus if contracted can be detected after 72 hours (third day) of high fever or other related symptoms suspected for dengue fever.
Mr. Liyanage said the SPC would not suffer any financial loss because in terms of the agreement with the supplier, the kit was to be sold on a commission basis. “We agreed to the deal because it was approved by the NMRA,” he said.
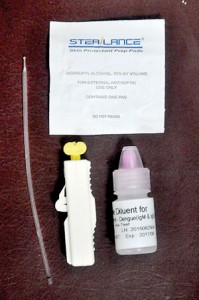
The kit was sold at Rs. 620 at Osu Sala outlets and Osu Sala franchise holders throughout the country
National Dengue Prevention and Control Programme Chief Dr. Haritha Tissera, who is against the sale of the dengue kit, said that following objections raised by the Infectious Diseases Hospital (IDH), the Health Ministry’s epidemiology unit had recommended that the sale of the dengue detection kit be frozen immediately.
Consultant Pediatrician Dr. Lakkumar Fernando who was present at the launch of the kit said NMRA Chairman, Prof. Asitha de Silva had consulted him last week to discuss the issue and told him that it was not advisable to sell the kit directly to the public because based on the test results they may wrongly conclude that they do not carry the virus and would not seek medical help.
He said anti-bodies were not formed until five to seven days of the infection and if medical help was not sought in time, it would be too late to save the patient.
Dr. Fernando said he attended the DRDK launch because he thought the company was to supply the antigen kit.
He said antigen tests were reliable and could detect the virus within 20 hours of infection.
Infectious Diseases Hospital (IDH) Chief Dr. Ananda Wijewickrema said he lodged an objection with the NMRA last week as the efficacy of the kit was highly questionable and therefore could lead to fatalities.
He explained that the antibodies for Dengue Hemorrhage Fever and Dengue Fever (DHF/DF) were formed only after around the fifth day and doing the test on the third day (72 hours) would not detect the disease. “This makes them believe the symptoms are indicative of a benign flu and delay seeking medical help,” he said.
 The National Medicinal Drugs Authority (NMRA), it is understood, had initially given one year provisional registration for the DRDK and that the time period had lapsed. The product does not appear in the Cosmetic Devices and Drug Regulatory Authority registry but the company is continuing to import the product on a no objection letter issued by the NMRA.
The National Medicinal Drugs Authority (NMRA), it is understood, had initially given one year provisional registration for the DRDK and that the time period had lapsed. The product does not appear in the Cosmetic Devices and Drug Regulatory Authority registry but the company is continuing to import the product on a no objection letter issued by the NMRA.
NMRA Chief Executive Officer Kamal Jayasinghe said the company might have placed the order for the consignment before the expiry of the registration and that the usual practice was to issue ‘no objection letters’ for clearance of cargo that have already arrived in the country.
Meanwhile, the World Health Organization on its website states that IgG antibodies in DHF are detectable only at low level by the end of the first week and then increases subsequently. Serological tests based on antibody done during the first five days of clinical illness are usually negative.
WHO believes that the tests are uncertain as they have not yet been properly validated. It said rapid tests could yield false positive results because of cross-reaction with other virus and serous samples taken in the first five days on the onset of illness and will not have detectable IgM antibodies.

