News
NMRA responds to story on withdrawal of essential drugs
In response to The Sunday Times Page 1 story last week on the possible withdrawal of essential drugs, the National Medicines Regulatory Authority has issued the following statement:
The title of the article ‘Many essential drugs being withdrawn from Sri Lank (14 October issue) itself is misleading. It appears the writer aimed to
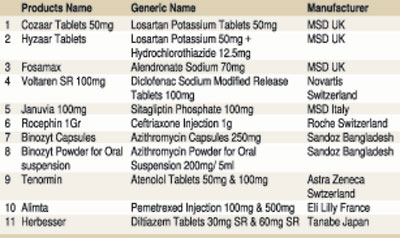
sensationalise a poorly researched article on an important matter by stating ‘many essential drugs’ when in fact she is referring to the possibility of a few expensive brands of medicines used by an extremely small percentage of patients being withdrawn from Sri Lanka. The writer has deliberately ignored the fact that there are many other good quality brands of the essential medicines referred to in the article. For example, tenormin, a trade preparation (brand) of a drug she has mentioned as being withdrawn is the most expensive brand of the drug atenolol. There are many other brands of the same drug widely available in the market.
The news item goes on to boldly state “Sri Lanka would be left with substandard and untested drugs which would cause prolonged illness and death in many a patient’ due to price regulation of essential medicines.” This dangerous and mischievous assertion is based on the possibility of some brands of drugs with insignificant sales volumes and market shares being withdrawn from the market due to their high cost.
- The primary objective of the NMRA is to increase patient access to quality-assured medicines. Cost is a major determinant of access to medicine.
- An expert report on Sri Lanka’s pharmaceutical market compiled by a WHO consultant in 2015 highlighted numerous unethical practices of pharmaceutical importers to leverage the supply chain including ‘loading’ of CIF prices and provision of bonuses and rebates to distributors. As a result, the report concluded the benefit of tax and duty exemptions provided to medicines by the government does not get passed on to patients.
- Pursuant to this report and a number of public complaints regarding exorbitant prices of medicines, the NMRA initiated price regulation of medicinal products to provide relief to patients in October 2016. Contrary to what has been alluded to in the referenced column price regulation of medicines is done after extensive study by the Pricing Committee of the NMRA as mandated by the NMRA Act No5 of 2015. This committee comprises medical and public health specialists, economists, an accountant representing the Association of Charted Certified . Accountants (ACCA) and representatives from the Consumer Affairs Authority and the Ministry of Finance. A very careful analysis of retail prices, market shares and trends of medicines is done before making recommendations to the Hon. Minister of Health who is the ultimate authority on pricing of medicines. This is followed by a consultation process with industry representatives and others before the relevant gazette notification is issued by the Hon. Minister.
- In line with WHO guidelines on pricing of medicines, the NMRA is committed to increasing patient access to affordable medicines by increasing the availability of quality-assured generics and biosimilars. The resultant competition in the market creates the required environment to regulate prices through many interventions including the maximum retail price model.
- The NMRA to date has regulated prices of more than 70 medicines including very expensive drugs used to treat cancer as well as devices such as cardiac stents and intraocular lenses. This has brought significant relief to our patients and the cost of a standard prescription of drugs used to treat a patient with diabetes and high blood pressure has been reduced by approximately 60%. Analysis of market data obtained from International Marketing Services (IMS) clearly shows a significant increase in the use of medicines after price regulation. For example, the use of a widely prescribed formulation of metformin used for the treatment of diabetes mellitus and rosuvastatin used for the treatment of high blood cholesterol has increased by 26% and 21% respectively over a period of one year from 2016 to 2017. A similar pattern is seen with many other medicines including widely-used antibiotics that indicates greater patient compliance to prescription due to increased affordability.
- The World Health Organisation in its annual report of 2017 commended the NMRA’s revised pricing policy of medicines “as a major achievement in safeguarding patients’ rights to access affordable medicine in Sri Lanka”.
- The said news item has highlighted the possible withdrawal of some brands of medicines. These brands (e.g. januvia, cozaar, binozyt, tenormin, etc) have a miniscule market share for the relevant medicine in Sri Lanka. We have enumerated in the table below market shares, both in terms of sales volume and percentage, of these brands in comparison to the overall sale of the respective molecules from 2014 to 2017 obtained from the IMS database for your easy reference. For example, market shares of brands such as januvia, cozaar and binozyt from the total market share of the relevant drug, namely sitagliptin, losartan and azithromycin in 2015 respectively were 0.52%, 0.1% and 0.07%. To illustrate this further, in 2015, cozaar had a sale volume of 155,792 tablets out of the overall losartan sale of 144 million, just 0.1% of the overall market.
- Clearly, the great majority of patients in our country are not prescribed these brands and their very insignificant market shares predate price regulation by the NMRA. Obviously, many other good quality brands of the referenced medicines which are more widely prescribed are available for the benefit of our patients.
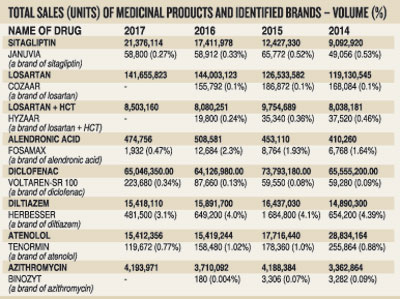
Completely disregarding these facts, the news item in the Sunday Times makes the baseless claim that essential medicines will be withdrawn from Sri Lanka based on the possible withdrawal of some selected brands of medicines that have in most cases a market share of less than 1% as illustrated in the table.
We also wish to state that the NMRA has repeatedly requested the Sri Lanka Chamber of the Pharmaceutical Industry (SLCPI) to provide verifiable and accurate CIF (cost, insurance & freight) prices of medicines imported by them to develop a reasonable pricing formula. Unfortunately, this has not happened. Therefore, without establishing the real cost of a medicine imported to the country, which the importers seem reluctant to divulge, the NMRA is unable to develop such a formula.
The news item concludes by stating the “NMRA was not contactable for comment”. This is a complete falsehood. The writer did not make any credible effort to contact the NMRA to verify the information she had purportedly received from various unidentified “sources”.
| Association of Medical Specialists fully supports the Health Minister | |
| The Association of Medical Specialists (AMS) says that as the organisation that represents the majority of Medical Specialists in the state sector, it believes it has a duty to stress to the people that the news item on the front page of the Sunday Times last week and carried by some Sinhala newspapers during the week that “Sri Lanka would be left with substandard and untested drugs which would cause prolonged illness and death in many patients as a result of recent price regulations” is largely untrue and misleading. The AMS agrees and endorses the facts mentioned in the detailed reply issued by the National Medicinal Regulatory Authority (NMRA) on October 19 in response this story. It was sad to note that this article tried to implicate ‘some doctors’ among the unnamed sources it refers to, and the AMS would like to totally dissociate with the claims made in the name of ‘doctors’ in this article titled ‘Many essential drugs being withdrawn from Sri Lanka”. The AMS wishes to commend and fully support the Hon. Health Minister’s initiative and mission of price regulating essential drugs and devices to increase patient access to quality-assured medicines. We also agree that cost is a major determinant of access to medicine, and we too totally agree that the price reductions of essential drugs done during the past two years has brought significant relief to many patients and there is a clear increase in the use of high quality drugs or better brands now than before as those drugs have become more affordable as clearly shown in NMRA’s detailed reply. Some brands with high price time to time leaving the market due to poor sales they had amidst the competition they faced when alternatives were available, at a more affordable price, occurred even before price regulations started, and we would like to stress that there is no reason for panic even if the brands mentioned in the article leave our country as quality alternatives of them are already available, as shown in NMRA’s reply. |