News
Breakthrough in quest for anti-viral drug for COVID-19?
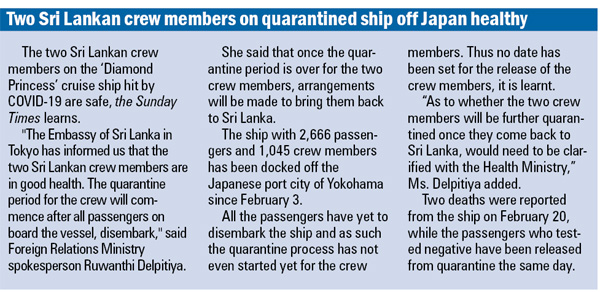
A study by scientists attached to the University of Colombo has pointed the way towards possible medications that could be used in the battle against the new coronavirus.

Someone to lean on: The patient with her 'guardian angels' as she leaves the National Institute of Infectious Diseases (NIID), Angoda. Pic by Sameera Werasekera
“Our study will go online today after which it will be picked up by a scientific journal,” Dr. Sameera R. Samarakoon of the Institute of Biochemistry, Molecular Biology & Biotechnology (IBMBB) of the University of Colombo told the Sunday Times.
Instead of trying out untested compounds, Dr. Samarakoon and team members Prof. Kamani Tennekoon and Dr. Kanishka Senathilake had decided to look at the tested compounds for diseases already approved by the Food and Drug Authority (FDA) of America.
“There are about 3,500 such compounds and we got four hits,” says Dr. Samarakoon with excitement, hoping that Sri Lanka could work with countries such as China and America in taking it forward by initiating trials in a clinical setting.
In a different turn of events on Wednesday, at the National Institute of Infectious Diseases (NIID), Angoda, it was time for smiles, hugs and selfies as the Chinese woman tourist who was the single ‘imported’ case of the new coronavirus in Sri Lanka left for China.
Greeted with a bouquet of flowers by Health Minister Pavithra Wanniarachchi and top officials including the Director-General of Health Services, Dr. Anil Jasinghe, she placed a tray of flowers at the Budu Medura, before being discharged from the hospital. She communicated with those present through an App which acted as the translator.
NIID’s Consultant Physician Dr. Eranga Narangoda told the Sunday Times that he and his team looked after the Chinese patient from January 25 until February 19, when she left for home in China. (See box)
Off Thurstan Road, meanwhile, seated within the IBMBB building Dr. Samarakoon and his team were on a different track.
Dr. Samarakoon who is engaged in testing anti-cancer compounds was giving thought to adapting the set-up already in place for such testing, for the urgent need of fighting the disease – COVID-19.
Before detailing the study and the modus operandi of viruses, he points out how there is a rush to find a vaccine rather than testing compounds to produce anti-viral medication. This is because finding anti-viral medication is time-consuming and may yield results only in about 5-10 years.
So, what Dr. Samarakoon did was – without trying out new and untested compounds which would have to go through the rigorous processes of testing for toxicity etc – pick up the FDA-approved drugs which had already gone through stringent testing.

Prof. Kamani Tennekoon

Dr. Sameera R. Samarakoon

Dr. Kanishka Senathilake
The testing before a new drug is introduced includes the initial step of using computer models. If it passes the first stage, then testing is done on cell cultures and animals in a pre-clinical drug trial. If it passes those as well, the drug would be used in human clinical trials. New drugs need to be tested and trialled for safety, effectiveness and dosage before they can be prescribed to patients.
Next, Dr. Samarakoon opens up the complex world of molecules and RNA (ribonucleic acid) and explains that Chinese scientists had come up with the viral genome (RNA) of the new coronavirus.
The genome in the new coronavirus (named SARS-CoV-2) is a complete set of RNA which contains all the information needed to build and maintain it. In humans, a copy of the entire genome, more than 3 billion DNA base pairs, is contained in all cells that have a nucleus. While in humans, these cells can divide by themselves and grow, in viruses they cannot reproduce by themselves.
Dr. Samarakoon explains how a virus, when it enters (infects) a susceptible human cell can direct that cell machinery to produce more viruses, each consisting of nucleic acid and an outer shell of protein.
Viral replication involves ‘attachment to a host cell, penetration, uncoating, replication, assembly and release’. During ‘attachment and penetration’, the virus attaches itself to a host cell and injects its genetic material into it; during ‘uncoating, replication and assembly’, the viral RNA incorporates itself into the host cell’s genetic material and induces it to replicate the viral genome. During ‘release’, the newly-created viruses are released to the other cells of the host.
Spotlighting what they did in the IBMBB laboratory, Dr. Samarakoon says how they made a ‘model’ of the virus protein which is involved in causing the infection process in humans. Thereafter, they set about trying to block that activity, using the FDA-approved compound list.
“We did the computational analysis and found that four can inhibit or bind with the protein to stop its infective process,” he said, adding that a few compounds have good affinity with the protein.
“Yes, we believe it is a breakthrough,” adds Dr. Samarakoon.
| How NIID managed the patient | |
| Explaining the management of the Chinese woman tourist who was detected with the new coronavirus (COVID-19 disease), NIID’s Consultant Physician Dr. Eranga Narangoda said that her symptoms were fever, phlegm and body aches and pains. She had also lost her appetite.  Dr. Eranga Narangoda “Admitted to Ward 4, initially we treated her with an anti-viral medication but when on the 4th day there was a worsening of her symptoms, we were a little worried and started her on a five-day antibiotic regimen,” he said pointing out that sometimes a viral infection is followed by a bacterial infection and the antibiotic was for that. The patient became asymptomatic (not showing symptoms) by the 5th day. She was discharged only after two tests conducted by the Medical Research Institute (MRI) came negative indicating that she was clear of the virus, it is learnt. |
| State-of-art technology at National Influenza Centre can detect 16 viruses in one go | |
| Sri Lanka now has state-of-the-art PCR (Polymerase Chain Reaction) technology to detect many viruses in “one go” rather than going through the tedious process of testing one virus after another which is also very time consuming.  Dr Jude Jayamaha “We can test for 16 viruses simultaneously,” revealed Consultant Medical Virologist Dr. Jude Jayamaha of the National Influenza Centre, Department of Virology, Medical Research Institute (MRI), Colombo. He lists the 16 viruses as: Influenza A & B; Respiratory Syncytial Virus (RSV) A & B; Parainfluenza 1, 2, 3 & 4; Adenovirus; Human metapneumovirus A & B; Rhinovirus; and the human coronavirus 4 types. This assay is very useful in detecting an outbreak (a cluster) in the early phase or respiratory distress/atypical pneumonia even in a severe case, he said, explaining that PCR technology is widely used in molecular biology to rapidly make millions to billions of copies of a specific DNA or RNA (like in the case of the new coronavirus) sample, allowing scientists to take a very small sample and amplify it to a large enough amount to study in detail. As per the World Health Organization (WHO) recommendations, samples from patients suspected to be struck down by COVID-19 were tested with this panel, he added. |