News
Lessons of COVID-19 that go beyond health

From left: Prof. Indika Karunathilake; Prof. Vajira H.W. Dissanayake; Dr. Sunil De Alwis; Dr. RaziaPendse; and Prof. M.C. Weerasinghe at the inauguration, listening to Prof. Malik Peiris.
The image had been taken from one of the Apollo flights from near the surface of the moon, showing the earth and so came the explanation and the plea from distinguished expert Prof. Malik Peiris………“You can see the barren wasteland of the moon and that beautiful planet that we call earth. We have to realize that this is a precious resource and in terms of public health, in terms of human wellbeing, we really have to pay attention to safeguarding our fragile ecosystem.”
Prof. Peiris’s powerful virtual presentation was followed by a poignant rendition of ‘DannoBudunge’ by Dr. Nilanka Anjalee Wickramasinghe, creating in the mind’s eye of the participants, Sri Lanka’s own little bit of earth – ‘Manelnelum ha oolupushpaadi…….serupanthipanthipiinathibohose……Anuradhanagarayadanpenenase’.
Tuesday morning was the inauguration of the five-day Annual Conference 2020 of the Asia-Pacific Academic Consortium for Public Health (APACPH), on the theme ‘Public Health in the New Normal’, co-hosted by the Sri Lanka Medical Association (SLMA) and the Faculty of Medicine, Colombo.
APACPH is an international academic organization comprising many largeand influential schools of public health in the Asia-Pacific region and is dedicated to improving professional education for public health.
While the APACPH President is Prof. Wah Yun Low of Malaysia,its Vice President Prof. IndikaKarunathilake and his team stepped in to host this key event in the APACPH calendar as a virtual conference due to the COVID-19 pandemic.
The Chief Guest Prof. Malik Peiris, Professor and Chair in Virology at the School of Public Health, Hong Kong University (HKU) and Co-director of the WHO H5 Reference Laboratory at HKU joined the conference virtually. Those gracing the head table in Sri Lanka were Prof. IndikaKarunathilake; the Dean of the Faculty of Medicine, Colombo, Prof. Vajira H.W. Dissanayake; the Health Ministry’s Additional Secretary Dr. Sunil De Alwis; the World Health Organization’s Representative to Sri Lanka, Dr.RaziaPendse; and SLMA Vice President Prof. M.C.Weerasinghe.
Prof. Karunathilake recalled how Sri Lanka hosted the APACPH conference in 2012 and handed over the reins to Wuhan, China.
“Nobody expected the current events. That was the time of SARS-CoV-1 in many countries,” he said.
Professor Malik Peiris is professor and chair in virology at the School of Public Health at The University of Hong Kong. He is a clinical and public health virologist with a particular interest in emerging virus disease at the animal-human interface, including influenza, coronaviruses (SARS-CoV, MERS-CoV, SARS-CoV-2) and others. His research encompasses the pathogenesis, innate immune responses, transmission, ecology and epidemiology of human and animal (poultry, swine, wild birds) influenza viruses. His collaborative research has provided understanding on the emergence and pathogenesis of the 2009 pandemic H1N1 virus and on avian influenza viruses H5N1, H9N2 and H7N9. Using a “one health”approach, these studies have provided evidence-based options for the control of these viruses in poultry and in humans. In 2003, he played a key role in the discovery that a novel coronavirus was the cause of SARS, its diagnosis and pathogenesis and contributed to its control. Recently he is researching the recently emerged MERS coronavirus and more recently, the newly emerged SARS-CoV-2.
He co-directs the WHO H5 Reference Laboratory at HKU and the HKU-Pasteur Research Pole, one of the network laboratories of the International Pasteur Network. He was elected a Fellow of the Royal Society of London in 2006, Fellow of the American Academy of Microbiology in 2016 and Foreign Associate of National Academy of Sciences in 2017. He was awarded the Officier de la Legion d’Honneur, France (2017), Mahathir Science Award, AkademiSains, Malaysia (2007) and Silver Bauhinia Star (S.B.S.), Hong Kong SAR (2008).>
Picking up the theme of the day, keynote speaker Prof.Peiris said that COVID-19 will be here probably in the next year or two.
His main point was that “we need better preparedness to confront emerging infectious diseases. When faced with a novel pandemic, as this one, in the initial stages, non-pharmaceutical public health interventions are all we have. But our knowledge on the mechanisms on respiratory virus transmission and the effectiveness of non-pharmaceutical intervention is still sub-optimal and poor.
“I think we really need to pay more attention to the science base behind this evidence. The value of diagnostics and how we can best use them in terms of achieving public health goals, the importance of communication and rebutting miscommunication that is taking a hold.”
Focusing on the science underlying public health interventions, since he is a scientist and virologist, Prof. Peiris quoted from an article in the prestigious ‘Science’ journal.
“The pandemic which has just swept round the earth has been without precedent. There have been more deadly epidemics, but they have been more circumscribed (for example Ebola in West Africa); there have been pandemics almost as widespread but they have been less deadly (for example the H1N1 pandemic of 2009). Floods, famines, earthquakes and volcanic eruptions (I might add tsunamis) have all written their stories in terms of destruction almost too terrible for comprehension, yet never has there been a catastrophe at once so sudden, so devastating and so universal.”
But, pointed out Prof. Peiris, this was not written in 2020, about COVID-19. It was written about the 1918 Spanish flu pandemic, over a hundred years ago. It is even more interesting to read the rest of the article where the author writes about the factors that stand in the way of prevention.
The factors read as:
n Public indifference which is part attributed to the confusion by the range of severity of the disease, ranging from asymptomatic to mild to fatal.
n Identifies the infection as being projected into the air, polluting the hands and environment unconsciously, invisibly and unsuspectingly, exactly what we have today in COVID-19.
n Prevention devolves on the infected person to prevent spread; those vulnerable can do little to protect themselves.
n The disease is transmissible before the person is aware he is infected.
Prof. Peiris said these are the comments that were made, which remain true up to today over a vast pandemic of comparable scale that happened over 100 years ago. I suppose it is also useful to reflect humbly, that although the last 100 years have seen so much of advances in medical science, vaccines, antibiotics and so many other aspects, still a simple virus can effectively bring the world to a standstill, as COVID-19 has done.
Looking at a graph, Prof.Peiris showed the total number of virologically confirmed COVID-19 deaths globally (as of December 5) which was 1.5 million and compared the numbers with the estimates of annual deaths for seasonal influenza, which range from 200,000 to 100,000. There are people who say that ‘Oh, COVID-19 is just another flu’, but it is not just another flu. These estimates for influenza are not based on virologically confirmed cases. If it were, they would be a tiny fraction of this 200,000. These estimates of influenza mortality are based on statistical modelling.
In his presentation, Prof. Peiris stated:
Now, I’m sure the true case fatality associated with COVID-19 is much greater than 1.5 million and, of course, as you know, there are factors that affect regional mortality, such as the age structure of the population, and in terms of reporting under-diagnosis.
I want to focus on just a few examples of the scientific basis for policy. So first, let’s look at the infectiousness profile of this virus, SARS-CoV-2, the cause of COVID-19.
In early February, we published this data, which we had accumulated in collaboration with colleagues in southern China, Guangdong, showing that the viral load profile in the upper respiratory tract in COVID-19 is maximal at the time of the onset of symptoms and then gradually declines from there onwards. It is very different from SARS (Severe Acute Respiratory Syndrome), where it was very low during the early four-five days and increased subsequently.
So, in that paper, we said that SARS-CoV-2 resembles influenza and will require strategies very different to those that were successful in the control of SARS-CoV-1. This is work from the epidemiologists from our School of Public Health and they were able to show, looking at a large cohort of patients from China, that about 40% or more than 40% of transmission takes place before the onset of clinicalsymptoms.The bulk of the rest of the transmission takes place in the next four or five days, after the onset of symptoms.
The damage is done before the patient even knows that he’s sick and the first few days after he developed symptoms. This is again confirmed by other studies, for example, where they have looked at contact tracing, again showing that transmission risk was greatest in the first five days or so after the onset of the infection.
This is the reason why containment of this pandemic is so difficult.
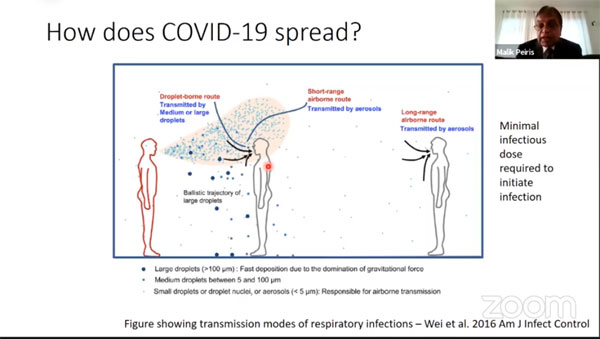
How is COVID-19 spread?
Our understanding of it is that it is primarily spread by the respiratory roots, large droplets that fall because of gravity within one or two metres and fine airborne particles that can spread for a longer distance. However, as you can see here (slide), as these large droplets or small airborne particles, leave the infected person’s mouth, they do get diluted. Therefore, distance plays an important role in the probability of getting infected.
You need a minimum infectious dose to initiate infection, a single virus particle is unlikely to initiate infection so that is important to understand.
Then, of course, there has been a lot of debate as to whether, in the case of coronaviruses, whether it is large respiratory droplets or fine aerosols that contribute to the spread of infectious virus. So, fortunately, we had been doing some studies on influenza in the previous years and some of those patients were not influenza, they were the seasonal coronaviruses and what you can see is that these patients when you monitor, their breath or coughing, they shed infectious virus both in the large droplet fraction and also the fine aerosol fraction.
Most importantly, when the same patients wore surgical masks, the number of infected particles leaving the respiratory tract and leaving their mouths was essentially close to zero.
This and the realization that COVID-19 is transmitted even before patients develop symptoms, that it is the pre-symptomatic phase and even from those asymptomatic individuals, this is the understanding that led to the recommendation for the use of surgical masks to be more widespread in the community.
Indeed now, there are epidemiological studies that support and provide evidence for the impact of the use of surgical masks or masks or face coverings of any sort in reducing transmission.
There is a study from Germany, where they have shown quite a substantial impact after the mandatory introduction of face coverings in reducing transmission.
In addition to airborne spread, there is also the potential for indirect transmission in the spread of COVID-19.
Slide from Prof. Malik Peiris's presentation
Virus survival on surfaces
So, we looked at the survival of the virus in the environment and what you can see here is the SARS-CoV-2 and we can see that on smooth surfaces such as stainless steel or glass or plastic, the virus survives and is infectious for many, many hours after being deposited there.
On the other hand, on material like wood, cloth or paper, the virus survival is much shorter, just a matter of minutes or an hour or so.
If you compare this long survival on surfaces with for example, influenza… influenza is dead within a matter of an hour of deposition on the surface, whereas COVID-19 and indeed SARS-CoV remains viable for many, many hours. The potential for indirect transmission is quite real.
Now, for indirect transmission to take place, you also have to have the virus surviving on the hands of the patient or the person. Now obviously, that’s a very difficult experiment to do ethically, but recently, colleagues from Japan have used some novel technology to essentially show that the survival of SARS-CoV-2 on the skin is much longer again compared to influenza. So taking these two together I would say, it would be providing circumstantial evidence for the possibility of transmission through fomites and through hands.
So just to summarize, we know the routes of transmission through the airborne route, through those contacts and through the indirect fomites. And as I pointed out, the issue of dose and dilution means that distance is important in reducing the risk and ventilation is important in reducing the risk of transmission.
There is evidence to show that face coverings reduce the risk of transmission. Although there is no direct evidence about hand hygiene reducing risk, the circumstantial evidence is very strongly in favour of the possibility of transmission taking place through hands and the importance of breaking transmission with hand hygiene.
Duration of infectiousness?
Now another question, of course, is the duration of infectiousness once a patient is diagnosed or once a patient is symptomatic, because this is important in terms of how long the patient needs to be isolated or hospitalized.
A study that we did in Hong Kong on patients and the viral load by PCR, we saw that some of these patients are shedding PCR positive virus for quite a long time, 40 days, 50 days, 60 days. What we found was that firstly, infectious virus is culturable only within the first eight or nine days after the onset of clinical symptoms and only in those specimens which have a very high viral load, over 1 million virus particles or so.
So this means that infectiousness is not equal to PCR. So PCR positivity does not necessarily mean that you’re detecting infectious virus.
It’s not just our study that has been done, but also a larger study in the United Kingdom, where essentially the same conclusions came that infectious viruses are found early in the illness and in patients with high viral load.
Of course, in patients who are severely ill or immune-compromised, they may be infectious for a much longer period of time. So it is this information that led to a change in policy of discharge in many countries including Hong Kong, Sri Lanka and the World Health Organization (WHO), where after 10 days or so after the onset of symptoms, once their symptoms are resolved, they can be discharged safely.
So this is important when you’re thinking about the policy of interrupting transmission, which is a different question if you like, from diagnosing and treating patients.
When you’re focusing on interrupting transmission and, for example, doing diagnostic tests to interrupt transmission in the community, this realization is important because what you really need is to find patients in this phase of the illness, those with high viral loads.
This is why although PCR is the most sensitive diagnostic test that we have, some of the better and for example WHO-approved antigen tests and rapid diagnostic tests do play a role.
There is another paper where they talk about the impact in terms of transmission on the sensitivity of a test, the turnaround time of results and the frequency of testing.
What they find in this analysis is that it is not the sensitivity of the test that is most important in terms of interrupting the process. So it’s important to understand that we need to use the different tests that we have available in an intelligent way to impact on the question that we are trying to address.
Vaccines
Now, of course, vaccines are on the horizon. It is very fortunate that it seems that many of these vaccines do seem to be effective, at least the three that have provided Phase 3 data so far. It is also useful to recapitulate previous viral vaccines. Classically, the approach to a viral vaccine has been to grow the virus, kill it and inject it. Examples are the polio and rabies vaccines.
The other approach to viral vaccines has been to attenuate the virus, to make the virus weaker so that it doesn’t cause disease, but it still can infect and/or induce an immune response. These include vaccines like measles, oral polio, mumps, rubella and varicella.
Then you have the vaccines based on individual components of the virus proteins. For example, Hepatitis B and HPV.
More recently, of course, there have been RNA vaccines where instead of injecting protein, we inject the mRNA that codes for this protein.And then of course, you can also inject the DNA that codes for the RNA that codes for the protein.
You can also use another approach to deliver the gene that makes the protein into the cell using a replication incompetent viral vector, such as the adenovirus that is being used in some of the vaccines.
These strategies are basically making the cell become the vaccine factory and it is quite important for communication because there’s a lot of misinformation out there.
It is important to be very clear that this RNA that is injected, will not get integrated into host DNA, the cell has no mechanism to convert RNA to DNA. So, there is no risk that this RNA is going to alter your DNA in any way. This seems to be misinformation that is going around.
So how do the COVID-19 vaccines fit into this framework?
The Moderna and Pfizer-BioNTech vaccines are RNA vaccines. The AstraZeneca, CanSino, Gamaleya and Janssen all use this adenovirus vector strategy. But importantly, they use different adenoviruses to deliver the protein RNA into the cell and there are differences, theoretical differences in these.
The problem is that if you use a human adenovirus like Adenovirus 5, many of us will have pre-existing antibodies which may compromise the success of that vaccine, whereas a chimpanzee virus, because humans have no prior immunity, is likely to be more successful.
The Phase 3 trials are the thing that will tell us the final answer. There are traditional vaccines such as Sinovac and there are protein vaccines coming along as well,Novavax and Sanofi GSK.
Having said that, and as I pointed out, it is really fantastic news that at least the first three vaccines have released data from Phase 3 trials that seem to be very promising. But we just need to think about what we can expect from this first generation of COVID-19 vaccines. It is important to note that the endpoints in these Phase 3 clinical trials are protection from disease, not prevention of transmission.
We cannot assume that a vaccine that protects from disease necessarily will equally protect from transmission. So, if we just look at this situation here, when you have a natural infection, you have the virus infecting your nasopharynx and your lungs, you will develop immunity in your nasopharynx and in your lungs. You’re likely to be protected from disease but also from infections in your nasopharynx and, therefore, prevent transmission.
If you have an injected vaccine, what you have is antibodies in the blood, which will likely protect you from severe disease, but it may or may not prevent virus colonizing and replicating in the nasopharynx.
This is why we need to be very careful when we are thinking about what you expect these first generation vaccines to do.
Now there’s a lot of talk about population immunity, what is popularly called herd immunity and as I pointed out, at the moment, there is really no evidence that the protection that we are seeing is extremely welcome and which will protect the most vulnerable in our population from severe disease.Whether this will translate into prevention from transmission and support is something to think about.
It’s not just the science, it’s not just the public health, we really have to think about social and behavioural aspects and behavioural science when we are trying to control a pandemic like this.
I won’t go into the details here, but as you can see the issues of communication, miscommunication, the social context, the importance of leadership and the perception in the community about the threat, are crucially important.
I think we need to learn from our behavioural, social science and anthropological colleagues on how best to communicate effectively.
Of course, again, a point that was made in one of the introductory comments, the need for strong public health systems. I think if nothing else, this pandemic has really shown why it is fundamentally important to have strong public health systems and, therefore, have an investment in building and maintaining these public health systems. But then, in the last slide (the image of earth), I just want to go beyond pandemics.
The pandemic COVID-19 is bad, it’s terrible. But ultimately, we will find the survivors. But I think we have much greater issues in front of us. What the pandemic has shown us is what science has been telling us, to expect such a drastic event such as a pandemic like this and policy-makers, politicians, economists ignored it until it was too late.
But there are other things that science is telling us and that is that we are living beyond our means in terms of planetary sustainability. In terms of ozone layer depletion, in terms of climate change, in terms of nitrification, in terms of biodiversity loss, in terms of alkalis and in nature, nothing grows forever.
This is a law of nature, and success, economic success, as defined as annual increase of GDP is unsustainable.
It makes no sense if you think about it. What we really need is human contentment, happiness and wellbeing. Not just a percentage increase in GDP year on year, assuming that this is benefiting our populations, because we really are at risk of rupturing the limits of planetary sustainability.
As you can see, examples of massive climate events, huge pollution, lossof our environment. I think COVID-19 is a great teaching opportunity to raise awareness that we cannot ignore the risks that science has been highlighting over the past few decades and this particularly applies to these issues of planetary sustainability that I was talking about.

