News
SL may face vaccine shortage for 2nd jab
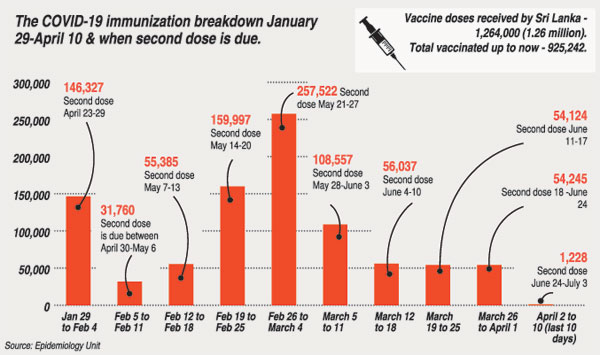
Will Sri Lanka have adequate vaccines to give the second dose to 925,242 people who have got the first jab between January 29 and April 10?
Health experts pointed out that if the country does not get another stock of Oxford-AstraZeneca vaccines the latest by mid-May, there will not be adequate vaccines as the in-hand doses of 338,758 will run out.

Dr. Nihal Abeysinghe
This is the worry gripping the country as the Avurudu festivities and lengthy holidays came to a close, with many people throwing caution to the winds and going about hither and thither with nary a thought for the basic COVID-19 preventive measures.
No face-masks nor one-metre distancing were seen, while hand hygiene too was non-existent.
Promises and assurances by many including the State Pharmaceuticals Corporation (SPC) that vaccines were due in March and then in mid-April turned out to be damp squibs, with no stocks arriving up to today. Attempts by the Sunday Times to contact the SPC failed.
The second dose for the first batch of frontline healthcare workers who got the first jab on January 29, is due on April 23. The vaccine rollout on January 29 began with the symbolic vaccination of Consultant Physician Dr. Ananda Wijewickrama who has been at the forefront of the battle against COVID-19 at the National Institute of Infectious Diseases (NIID), Angoda.
A very pertinent question raised by former Chief Epidemiologist Dr. Nihal Abeysinghe, in the imminent scenario of a vaccine shortage was: Who decided to vaccinate all and sundry above 30 years of age in a ‘limited vaccine setting’?
“We have still not got an answer to who decided to scrap the priority list given by the Health Ministry’s high-level National Advisory Committee on Communicable Disease (NACCD),” pointed out Dr. Abeysinghe, reiterating that this answer as to who changed the course of the vaccination programme should be provided by the Epidemiology Unit. This is because such technical decisions should usually be taken by the Epidemiology Unit in collaboration with the Health Ministry Secretary and the Director-General of Health Services.
Someone must take the responsibility for derailing this vital immunization programme, he added.
Others said that with the excuse of health officials at that time being that they wished to “prevent” COVID-19 transmission by administering the vaccine to those over 30, now with no flow of regular vaccine stocks, the country has been caught between a rock and a hard place.
Earlier, the priority list to prevent death and cover the high-risk categories was:
- Frontline healthcare workers and frontline security forces personnel engaged in COVID-19 work
- The elderly over 60 years of age
- Others with co-morbidities
This was in keeping with the Strategic Advisory Group of Experts (SAGE) on immunization of the World Health Organization (WHO) recommendations:
- Stage I when there are ‘very limited doses’ of vaccines available for 1-10% of the national population – health workers at high to very high risk of acquiring and transmitting infection, followed by older adults defined by age-based risk, specific to the country or region. Age cut-off to be decided at the country level.
- Stage II when there are ‘limited doses’ of vaccines available for 11-20% of the national population – older adults not covered in Stage I, then groups with co-morbiditiesor health states determined to be at significantly higher risk of severe disease or death.

Official oil anointing ceremony at Vidyalankara Pirivena, Piliyandala: Little heed to social distancing. Pic by Indika Handuwala
| SL’s vaccine status Sri Lanka’s drug regulator, the National Medicines Regulatory Authority (NMRA), has so far granted emergency-use listing to Oxford-AstraZeneca’s COVISHIELD manufactured by Serum Institute, India and Sputnik-V manufactured by Gamaleya Research Institute, Russia. The NMRA, meanwhile, has granted a waiver to the vaccine commonly called Sinopharm manufactured by the state-owned China National Pharmaceutical Group Corporation for the import of a donation of 600,000 doses. This vaccine has been given to 2,469 Chinese workers in Colombo, Puttalam, Kandy and Hambantota between April 2 and 8. Vaccines received and administered so far –
COVAX (the COVID-19 Vaccine Global Access) is a global initiative which works with vaccine manufacturers to provide countries worldwide equitable access to safe and effective vaccines. It is co-led by GAVI (the Vaccine Alliance); CEPI (the Coalition for Epidemic Preparedness Innovation); and WHO (the World Health Organization). | |