News
Loud and clear call for transparency in medical supplies situ

Head-table at the induction ceremony (from the left) SLACPT Immediate Past President Senior Prof. Asita de Silva; SLACPT President Senior Prof. Priyadarshani Galappatthy; Chief Guest & Health Ministry Secretary, S. Janaka Sri Chandraguptha; Guest-of-Honour & Dean of the Colombo Faculty of Medicine, Senior Prof. Vajira H.W. Dissanayake; and SLACPT Secretary Prof. Priyanga Ranasinghe
Simple was the ceremony but powerful, timely and evidence-based the recommendations delivered in these troubled times when Sri Lanka’s health sector is facing numerous challenges.
The absolute need for transparency and accountability in all processes including registrations, purchases, pricing and usage of medicines was one of the key recommendations put forward by the newly-inducted 5th President of the Sri Lanka Association of Clinical Pharmacology and Therapeutics (SLACPT), Prof. Priyadarshani Galappatthy on August 12.
Reiterating that there was an urgent need to review the annual pharmaceutical and medical supplies situation, rational usage and annual budgetary allocation, this Senior Professor and Chair Professor of Pharmacology at the Faculty of Medicine, University of Colombo, suggested the establishment of a unit for this purpose with a Technical Advisory Committee with members drawn from all professional colleges to review and provide advice.
With the theme of the SLACPT for 2023-2024 being ‘Equitably available, affordable, safe and quality use of medicines for challenging times’ Prof. Galappatthy further urged the setting up of an electronic system where all stakeholders would be able to obtain relevant data on medicines including on stocks, costs, purchases and registrations by the National Medicines Regulatory Authority (NMRA) through an online system.
She said: “Accurate forecasting of quantities required for hospitals, based on previous ‘actual’ consumption figures, is a must. The lists of donations also need to be updated frequently with the proviso that what Sri Lanka would accept would only be those adhering to requirements.”
In the audience at the SLACPT function, held at the auditorium of the Faculty of Medicine, Colombo, were high-level Health Ministry officials including Secretary S. Janaka Sri Chandraguptha and Deputy Directors General (DDGs) of the Department of Health Services as well as NMRA Chairperson Prof. S.D. Jayaratne. The function, commendably and setting a shining example, was held without sponsorship from any pharmaceutical company.
With regard to the “relatively young and small” SLACPT set up in 2015, Prof. Galappatthy said that it was committed to developing the field of clinical pharmacology and therapeutics in the country. It aimed to promote best practices in utilization of medicines for improved patient care, while playing an advocacy role on policy and regulation of pharmaceuticals.
Smilingly calling a ‘clinical pharmacologist’ a “jack of all trades”, she quotes an Oxford Don that clinical pharmacology entails looking at “all” aspects and use of drugs in humans.
Under a Health Ministry assignment, the SLACPT had addressed the vital issue of ‘Can we make medicines equitably available, affordable, safe and ensure their rational use’, in a review of the pharmaceutical supply system – ‘Challenges, barriers and recommendations’.
The review identifies the pharmaceutical supply process; pharmaceutical market; expenditure on pharmaceuticals & medical devices; financial capability of supplying the current list of pharmaceuticals; challenges and barriers to the supply process; while providing recommendations for an effective supply of pharmaceuticals and medical devices.
Prof. Galappatthy had carried out a desk review of documents, reports and literature, while analysing the data pertaining to pharmaceuticals and holding 10 extensive meetings with stakeholders – the NMRA, Medical Supplies Division (MSD), State Pharmaceuticals Corporation (SPC), manufacturers, state hospital directors and Treasury.
 Comparing and contrasting the ‘achievements’ in the pharmaceutical sector prior to the crisis, she says that the availability of medicines was ‘high’ (over 80%) or ‘fairly high’ (between 50%-80%) both in the public and private health sectors. They were also affordable to the lowest income group.
Comparing and contrasting the ‘achievements’ in the pharmaceutical sector prior to the crisis, she says that the availability of medicines was ‘high’ (over 80%) or ‘fairly high’ (between 50%-80%) both in the public and private health sectors. They were also affordable to the lowest income group.
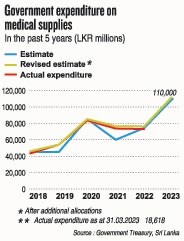
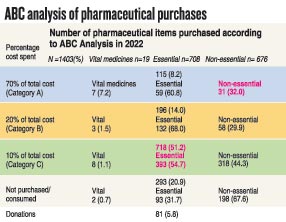
She stresses that the country needs a proper review of the current situation which is seeing severe shortages of essential medicines, as many as over 100 essential medicines being out of stock, as at March 2023.
Prof. Galappatthy has also looked closely at ‘Waivers of Registrations’ (WORs) in the past five years (2018 to 2022) which have caused much controversy in recent times. Here are her findings:
- 2018 – 136 WORs granted without a single rejection. Why 64 of these 136 WORs were granted was ‘unclear’ or not mentioned.
- 2019 – 270 WORs granted and 98 rejected. For 44 of the 270 WORs, the decision was unclear or not mentioned.
- 2020 – 185 WORs granted and a near-equal number (183) rejected. For 10 of the 185 WORs, the decision why was unclear or not mentioned.
- 2021 – 93 WORs granted and 208 rejected. For 19 of the 93 WORs, the decision why was unclear or not mentioned.
- 2022 – 98 WORs granted and many more, 130, rejected. For 12 of the 98 WORs, the decision why was unclear or not mentioned.
The 2023 list of WORs had not been given for analysis. However, the number of WORs is likely to be much higher than last year, according to Prof. Galappatthy.
“There are unprecedented challenges in the pharmaceutical situation in the country,” she says, urging action to prevent a reversal in the achievements in the health sector.
Pointing out that the SLACPT has provided numerous recommendations on how to improve the situation, Prof. Galappatthy adds that while it is supporting many activities to improve medication safety, “overall guarantees” for such an improvement are the responsibility of the Health Ministry and the government.
| Recommendations on pharma supply system handed over to Health Ministry | |
 Prof. Priyadarshani Galappatthy hands over her comprehensive review to Health Ministry Secretary S. Janaka Sri Chandraguptha Bringing to a close a major project of the SLACPT, Prof. Priyadarshani Galappatthy handed over to Health Ministry Secretary S. Janaka Sri Chandragupta a review, requested by the ministry, of the pharmaceutical supply system – ‘Challenges, barriers and recommendations’. The ministry had requested the expertise of the SLACPT in this regard. |
The best way to say that you found the home of your dreams is by finding it on Hitad.lk. We have listings for apartments for sale or rent in Sri Lanka, no matter what locale you're looking for! Whether you live in Colombo, Galle, Kandy, Matara, Jaffna and more - we've got them all!

